FDA certification rate for wearable ECG monitoring is nearly 50%, how will the market play in the next step?
2012 is “The first year of consumer wearables”, and 2018 is “The first year of medical wearables”. And for countries outside of China, 2017 is dubbed “The year of medical wearables”, since statistics showed that many of the FDA-approved dynamic ECG monitoring products were approved in 2017. At what stage of development is wearable dynamic ECG monitoring at home and abroad? After getting the authority certification, what are the next trends? Related industry insiders have found two major trends: 1.After breaking through the CFDA/FDA certification mark, the next step will be to connect doctors and diagnoses. 2.Bracelets and ECG stickers will be further integrated (one mountain can accommodate two tigers).
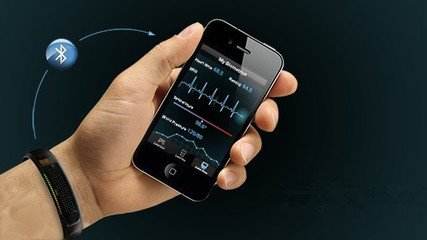
How will the dynamic ECG monitoring market break through the stock market and incremental market?
In the first year of medical wearable devices, wearable products began to play the role of disease screening and auxiliary diagnosis. For the four vital signs: body temperature, heart rate, blood pressure, respiratory rate have product breakthroughs, in addition, blood glucose, ECG, EEG, blood oxygen and other signs are also a battleground. Especially in heart rate monitoring, wearable devices can monitor atrial fibrillation (AF) and other hard-to-find arrhythmia. At present, Holter ECG wearable devices can more easily solve the medical needs of patients with arrhythmia. Similarly, some people who have symptoms of arrhythmia, such as palpitations, dizziness, shortness of breath, or unexplained fainting, may also choose to wear a dynamic ECG monitoring device. At the same time, there are some people who are trying to advance arrhythmia monitoring to asymptomatic patients, although at present it is controversial to define the high-risk group of atrial fibrillation and the general population. It is important to use data to demonstrate the benefits of ordinary people wearing dynamic ECG monitoring. Medical grade ECG monitoring products want to meet the needs of these two types of people. First, they must have accuracy and become an auxiliary diagnostic tool, but they must also be as smart, lightweight and friendly as a consumer product. At present, the entire US dynamic heart rate monitoring is a market of 1.4 billion US dollars per year. This market is still growing, considering that the launch of Apple Watch with ECG products will bring more consumer traffic. In our country, the number of sudden deaths due to heart disease each year exceeds 500,000. According to the "China Health and Family Planning Statistical Yearbook" issued by the National Health and Family Planning Commission, cardiovascular disease has become the leading cause of death among residents in China, higher than cancer and other diseases. The current dynamic ECG monitoring mainly adopts two kinds of signal collection methods: ECG and PPG. The ECG is detected by bio-electricity. After capturing the bio-electric signal and then digitizing it, then through digital signal processing, and then can output accurate and detailed heart health information. And PPG refers to photoplethysmography (PhotoPlethysmoGraphy), PPG for short. The principle of PPG to monitor heart rate is simple: the blood is red, reflecting red light and absorbing green light. PPG, through the detection of the amount of blood circulating at the wrist at a specific time, to obtain heart rate information. Another classification method is the difference in lead. A standard ECG requires 12 leads, 12-lead automatic ECG analysis system, capable of 12-lead, 6-lead, 3+1 lead, 3-lead, long-term rhythm lead record; and In a conventional ECG, if an abnormal heart rhythm is found, the 1-minute waveform recording and extension recording of the rhythm lead can be automatically completed. Recently, AliveCor announced the company's next direction: the introduction of a six-lead ECG compatible with smart phones. Perhaps one day wearable devices will also become medical monitoring products.

After breaking through the CFDA/FDA certification mark, the next step will be to assist diagnosis
In the consumer-grade wearable device bubble of 2012, healthy wearables fell into the predicament of homogenization and the collection of data. But now the entire market for wearable dynamic ECG monitoring equipment has begun to have new thresholds, with new rules of the game. There are traditional medical device companies as well as technology giants and startups are standing on the new starting line. After Apple released the Series 4 Apple Watch with ECG monitoring, Apple can be said to be the main player in this field. Apple's Series 4 Apple Watch ECG monitoring feature is FDA-approved. Apple Watch will issue a warning after monitoring the heart rate market. The user can then place the finger on the crown to collect the ECG signal. After 30 seconds, the ECG APP can be used to see the analog single-lead ECG. But there is no need to regard Apple as the number one enemy of wearable dynamic ECG monitoring, because Apple can promote atrial fibrillation monitoring to more consumer groups, who may not have a high risk of atrial fibrillation. From the short-term perspective, a large number of Apple users can provide a large amount of data to prove the benefits of dynamic ECG monitoring. In this study, 80% of the participants who wore ECG monitoring for the ECG function of the Apple Watch showed atrial fibrillation, and 98% of the ECG patches showed atrial fibrillation or other Clinically relevant arrhythmia. We can see that many products have already obtained FDA certification, and domestic AMAZFIT is also approved as a second-class medical device. After reviewing the certification mark, the next step is as important as getting doctors and getting users. This doctor may be a physical offline family doctor and may also have an AI to act as a diagnostic role. The domestic direction is to begin to combine a large number of health data with diagnosis and rehabilitation. In addition to the highlight of CFDA, this AMAZFIT App released by Huami is also worthy of attention. Arterial Network interviewed Lu Jigang, the founder of Ruihua Xinkang, a cardiac rehabilitation center providing medical support for Huami AMAZFIT, to learn more about how to turn a consumer medical grade bracelet into a “additional diagnostic tool”. In addition to online data integration and value mining, Ruihua Xinkang has also reached a strategic cooperation with Huami to create a closed loop of early screening, treatment and rehabilitation of heart problems. If the Huami bracelet is strong in hardware, then Ruihua Xinkang is helping to complete the layout of soft power.

One mountain will accommodate two tigers, and the bracelet and ECG will be further integrated.
We can also find that the main two forms are ECG stickers and smart watches, both domestically and internationally. The layout company adopting this product form is also the most. But many smart watch devices are not intended to provide a diagnosis, but simply tell the user if their ECG "shows signs of AF" Therefore, it does not replace the need for more explicit, physician-directed diagnostic monitoring, which requires equipment such as iRhythm. In fact, due to the wider coverage of the Apple Watch, the monitoring needs of iRhythm's Zio continuous 14-day ECG may increase. Although Apple's mobile phones, watches and applications will undoubtedly appeal to young and wealthy consumers, it remains to be seen how Apple will do to help people understand and manage serious health conditions such as AF. Coincidentally, in China, Huami's AMAZFIT also launched the bracelet and ECG at the same time. The AMAZFIT wearable Holter recorder supports both the wristband measurement and the chest-stick measurement. The use of the bracelet is similar to that of the Apple Smart Watch. In the wristband measurement mode, the user only needs to wear the ECG recorder on the wrist, keep the arm horizontally raised, and the other hand presses the index finger to the metal button at the bottom of the screen and then press on the top and hold the button to start measuring. The result can be obtained in about 30 seconds. Also worth noting is that more traditional device manufacturers choose to compete in this field with ICM (Implanted ECG Monitoring). However, ICM is costly, and implantable ECG monitoring is an interventional and invasive treatment that affects the patient's acceptance of it to some extent. In the field of ICM, Medtronic is the dominant player and the main product is Reveal LINQ. But now competitors not only have Abbott and Boston science, but also Apple. Abbott has launched the Confirm Rx device, while Boston Scientific is expected to launch ICM products in 2019. Abbott is currently the only ICM product for smart phones that does not require redundant transmitters.
